Experimentally observed photoluminescence (PL) or fluorescence spectra are often considered to give a direct view of microscopic electronic transition phenomena. The experimental setup of such kind of experiments is simple - at least in principle:
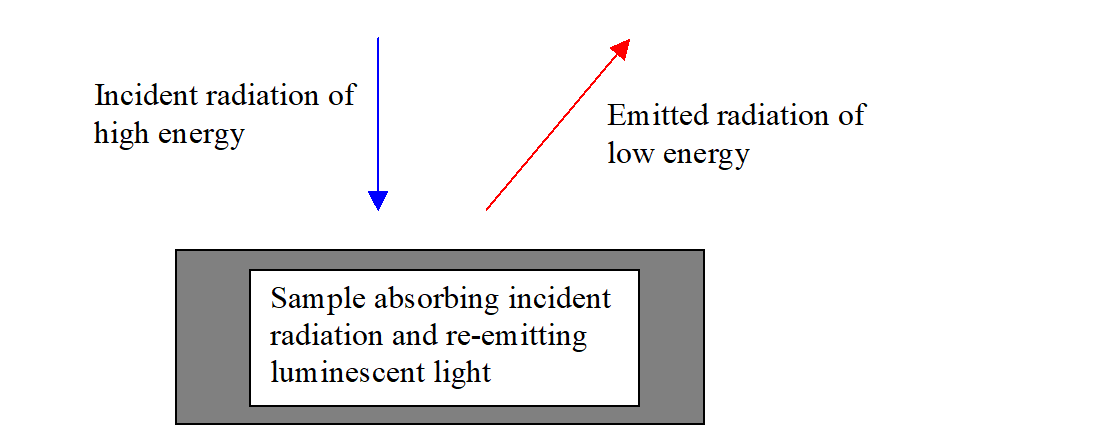
A schematic view of the photoluminescence process in the sample is this:
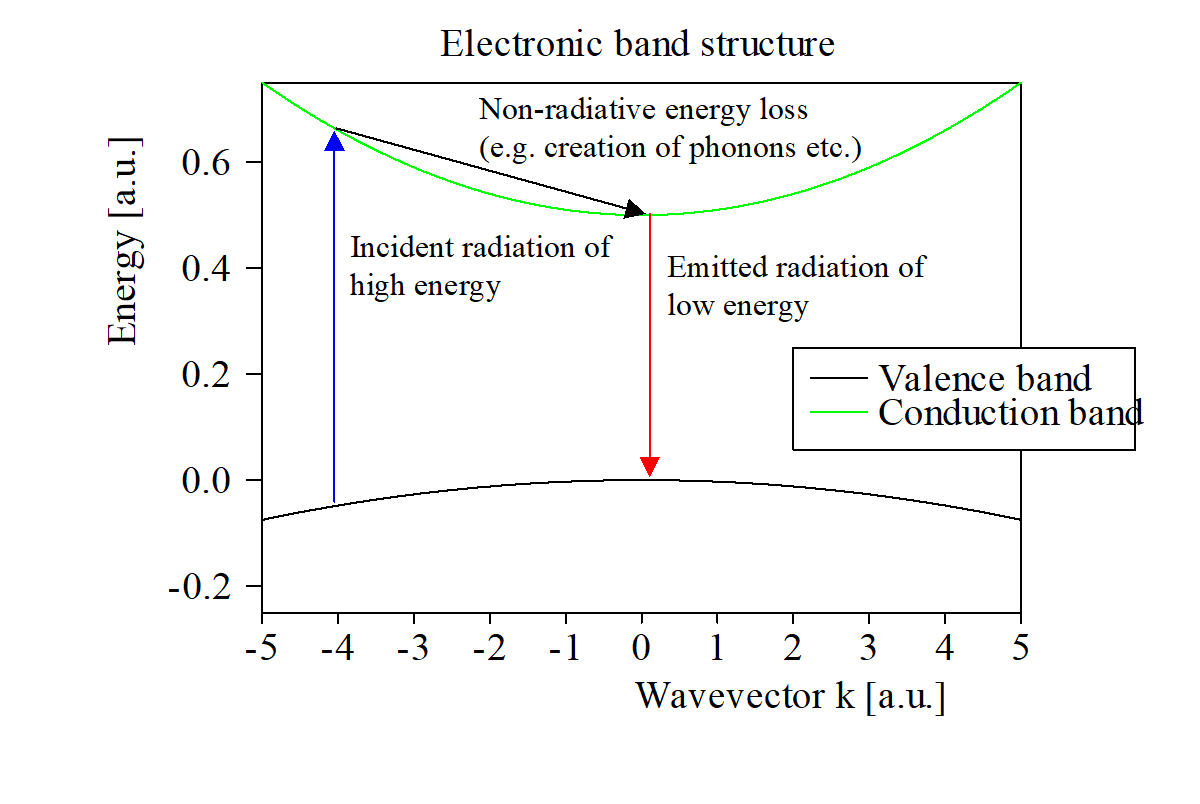
From this simple picture simple interpretation rules could be deduced. For example, the energy of the emitted radiation (or the energy of the peak of a PL spectrum) is the gap energy of the material (which can be identified this way). Or: If samples differ in intensity of the emitted radiation the non-radiative energy loss mechanisms are different.
However, usually life is more complicated, and before a microscopic interpretation of the observed luminescence spectrum is tried one should take into account all external phenomena which may influence the shape of the luminescence spectrum. In particular in thin film systems reflectance, interference and re-absorption phenomena can modify the shape of the PL spectrum significantly, and an intuitive interpretation can be quite misleading.
Knowing the optical constants of all materials in a layer stack, the SCOUT software can be used to compute the influence of all these external effects on PL spectra. This way a solid base for the correct interpretation of PL spectra is provided.
The following sections describe
•the algorithm to compute PL spectra
Examples of the analysis of measured PL spectra are given in a separate step-by-step PL tutorial (Tutorial 2, example 3).